38 parts of a helium atom
How many protons, neutrons and electrons does helium have? The helium atom contains about nine isotopes. Such as 2 He, 3 He, 4 He, 5 He, 6 He, 7 He, 8 He, 9 He, and 10 He. Among the isotopes, 3 He and 4 He are stable and are formed naturally. Number of neutrons through isotopes of helium The remaining isotopes of helium ( 5 He to 10 He) are highly unstable and their half-lives are very short. Build An Atom - Oak Ridge Institute for Science and Education After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the Periodic Table, explain elements, and have the background to understand isotopes. ... General questions about the properties of elements assume standard temperature and pressure (helium is liquid below ...
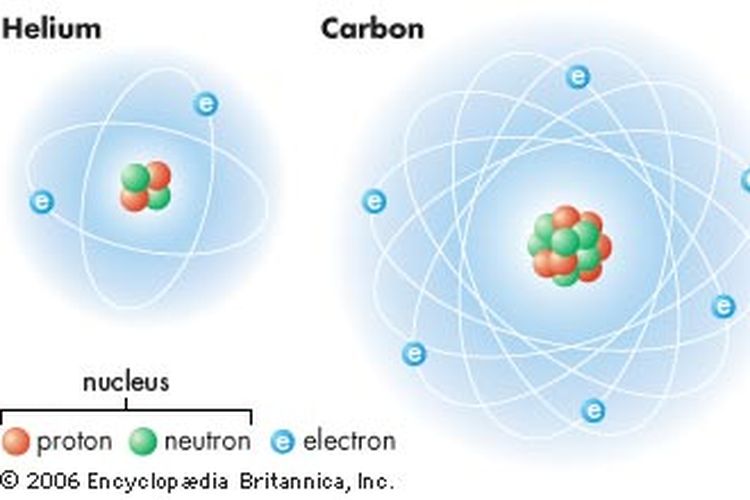
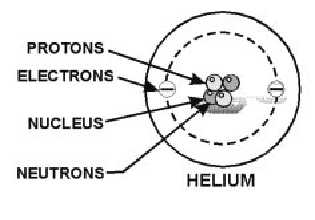
Sub-Atomic Particles - Chemistry LibreTexts A typical atom consists of three subatomic particles: protons, neutrons, and electrons (as seen in the helium atom below). Other particles exist as well, such as alpha and beta particles (which are discussed below). The Bohr model shows the three basic subatomic particles in a simple manner.

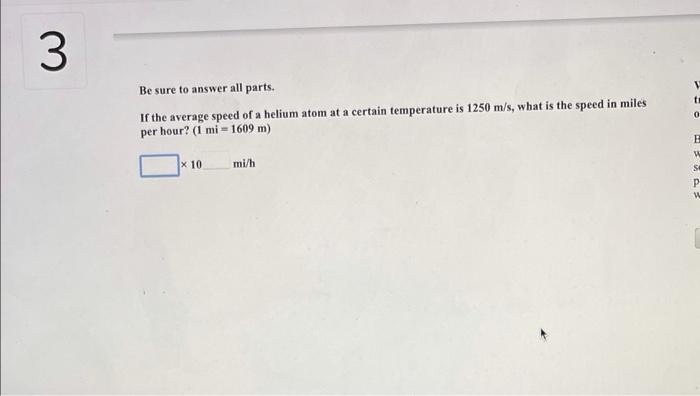
Parts of a helium atom
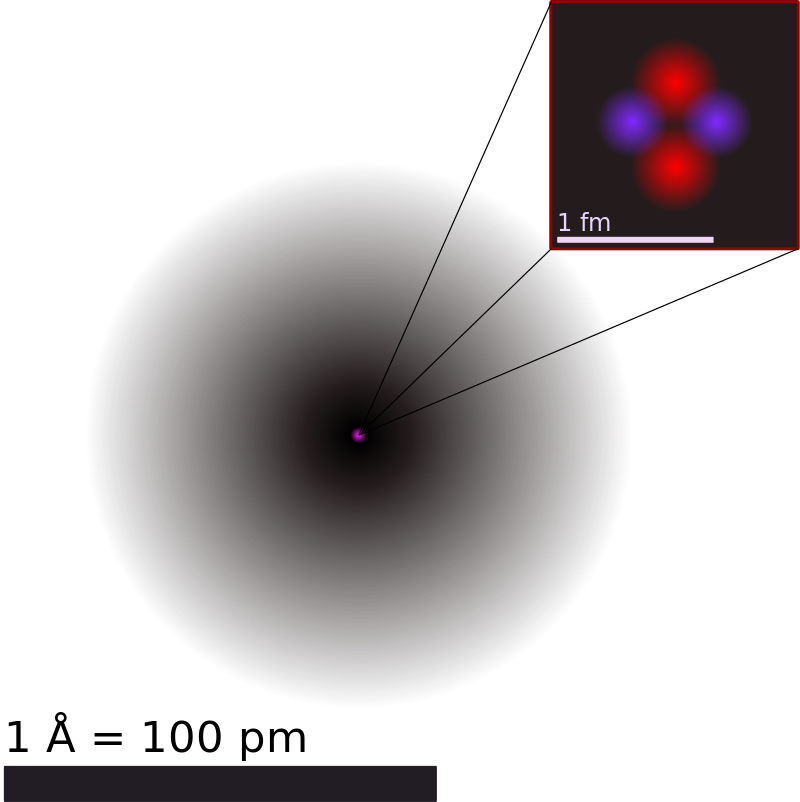
Helium Atom - University of Texas at Austin A helium atom consists of a nucleus of charge surrounded by two electrons. Let us attempt to calculate its ground-state energy. Let the nucleus lie at the origin of our coordinate system, and let the position vectors of the two electrons be and , respectively. The Hamiltonian of the system thus takes the form. (1180) A Heroic Effort to Measure Helium - Scientific American The muon kicks out both the atom's electrons, and what's left is the helium nucleus—which is two protons and two neutrons—and a muon. It's called a muonic helium ion. What Are The Parts Of An Atom? - Universe Today Structure Of The Atom: Our current model of the atom can be broken down into three constituents parts - protons, neutron, and electrons. Each of these parts has an associated charge, with...
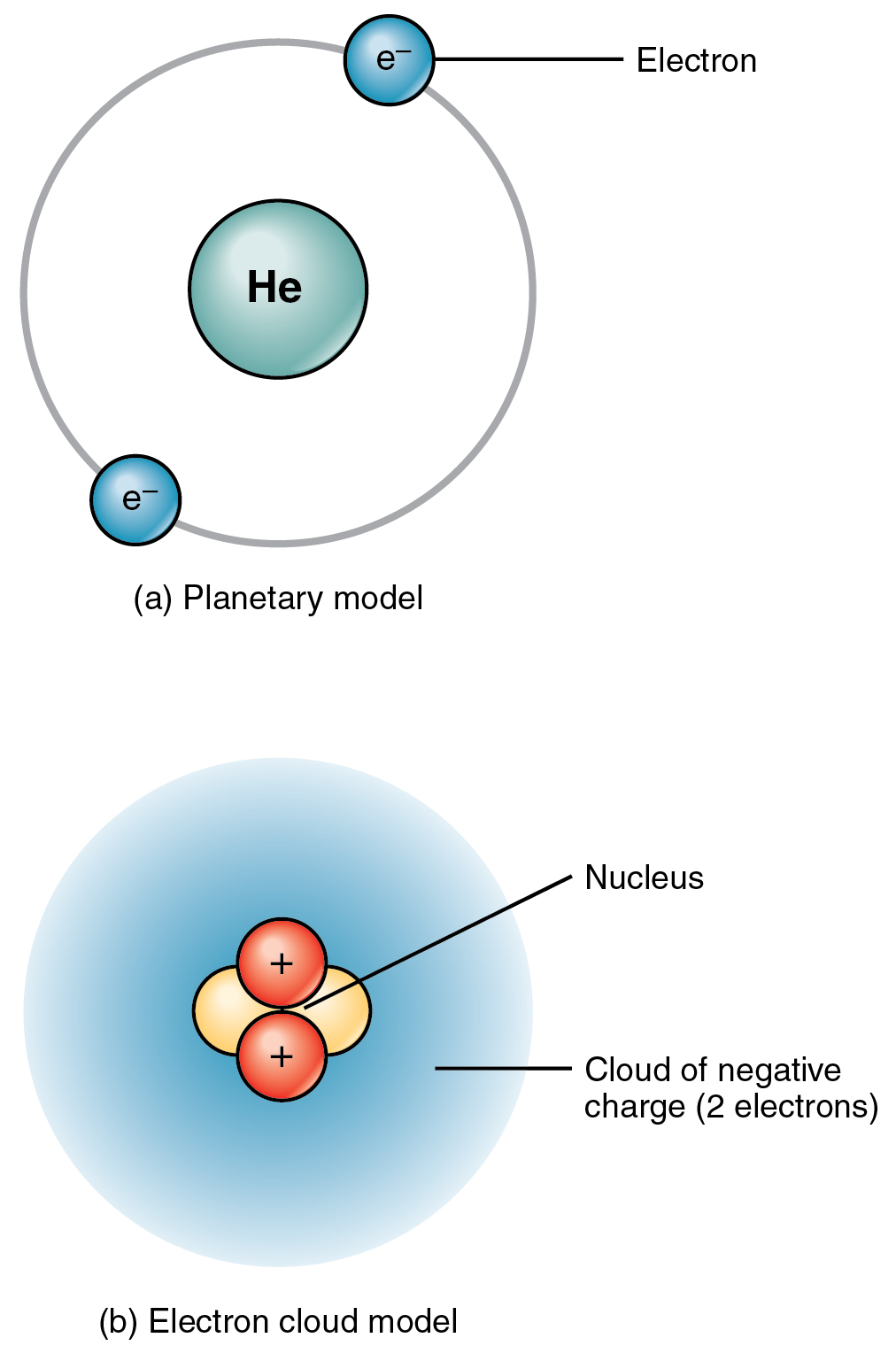
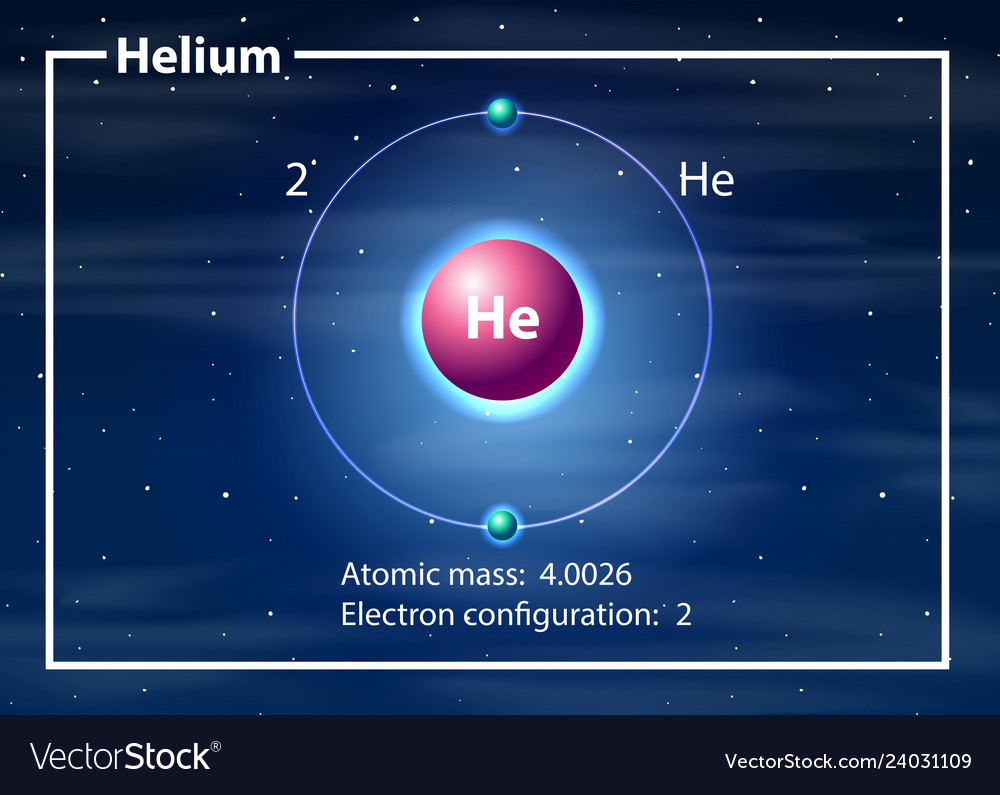
Parts of a helium atom. Dalton's atomic theory (article) | Khan Academy The first part of his theory states that all matter is made of atoms, which are indivisible. The second part of the theory says all atoms of a given element are identical in mass and properties. The third part says compounds are combinations of two or more different types of atoms. The Element Helium -- Helium Atom - World of Molecules Helium is a colorless, odorless, tasteless chemical element, one of the noble gases of the periodic table of elements. Its boiling and melting points are the lowest among the elements; except in extreme conditions, it exists only as a gas. The second most abundant element in the universe, significant amounts are found on Earth only in natural gas. Helium atom - Wikipedia A helium atom is an atom of the chemical element helium. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with either one or two neutrons, depending on the isotope, held together by the strong force. Periodic Table of Elements: Los Alamos National Laboratory Jules Cesar Janssen obtained the first evidence of helium. Diagram of a helium atom. There are only two electrons orbiting helium's nucleus. Helium ballons are lighter than air. Helium. Atomic Number: 2: ... The helium content of the atmosphere is about 1 part in 200,000. While it is present in various radioactive minerals as a decay product ...
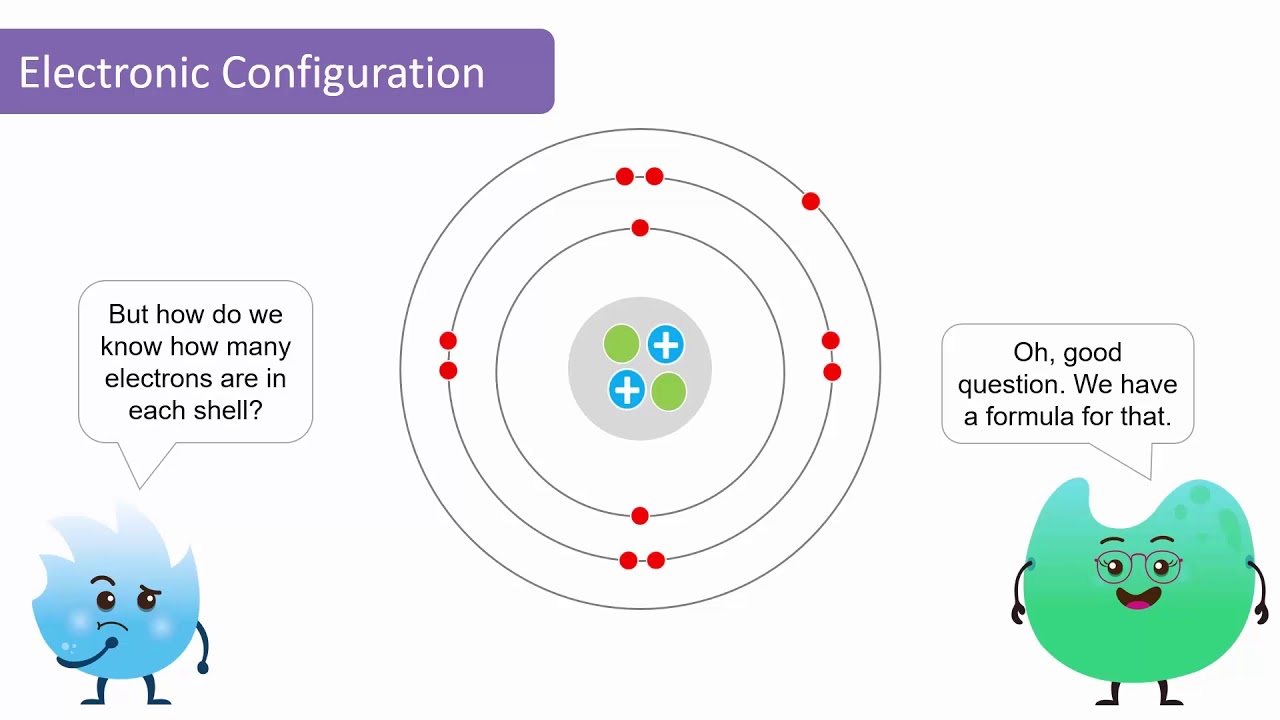
Atomic Structure Quiz Flashcards | Quizlet Draw and Label the parts of a helium atom. Include the mass and charge of each subatomic particle. Should have two protons and two neutrons and 2 electrons and an electron cloud. protons: +, 1 amu neutrons: 0, 1 amu electrons: -1, 1/1840 amu Explain the difference between the atomic number and the mass number of an atom. Helium - Element information, properties and uses | Periodic Table After hydrogen, helium is the second most abundant element in the universe. It is present in all stars. It was, and is still being, formed from alpha-particle decay of radioactive elements in the Earth. Some of the helium formed escapes into the atmosphere, which contains about 5 parts per million by volume. Helium | Definition, Properties, Uses, & Facts | Britannica Ordinary air contains about 5 parts per million of helium, and Earth's crust is only about 8 parts per billion. The nucleus of every helium atom contains two protons, but, as is the case with all elements, isotopes of helium exist. The known isotopes of helium contain from one to six neutrons, so their mass numbers range from three to eight. Helium - Periodic Table and Atomic Properties Helium is a chemical element with atomic number 2 which means there are 2 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
Helium Element | History, Uses, Facts, Physical & Chemical Characteristics Occurrence. Helium is present rarely on the Earth. However, it is the 2 nd most abundant element in the universe [2]. The atmospheric content of helium on the Earth is only 5.2 ppm [3]. There is a continuous production of helium on the Earth (via radioactive decay), but it readily escapes the Earth's atmosphere and enter the space. 8: The Helium Atom - Chemistry LibreTexts The helium atom has two electrons bound to a nucleus with charge Z = 2. The successive removal of the two electrons can be diagrammed as (8.1) He → I 1 He + + e − → I 2 He + + + 2 e − The first ionization energy I1, the minimum energy required to remove the first electron from helium, is experimentally 24.59 eV. Helium - Wikipedia Helium is composed of two electrons in atomic orbitals surrounding a nucleus containing two protons and (usually) two neutrons. As in Newtonian mechanics, no system that consists of more than two particles can be solved with an exact analytical mathematical approach (see 3-body problem) and helium is no exception. Matter, elements, and atoms | Chemistry of life (article) - Khan Academy An atom consists of two regions. The first is the tiny atomic nucleus, which is in the center of the atom and contains positively charged particles called protons and neutral, uncharged, particles called neutrons. The second, much larger, region of the atom is a "cloud" of electrons, negatively charged particles that orbit around the nucleus.
What Are The Parts Of An Atom? - Universe Today Structure Of The Atom: Our current model of the atom can be broken down into three constituents parts - protons, neutron, and electrons. Each of these parts has an associated charge, with...
A Heroic Effort to Measure Helium - Scientific American The muon kicks out both the atom's electrons, and what's left is the helium nucleus—which is two protons and two neutrons—and a muon. It's called a muonic helium ion.
Helium Atom - University of Texas at Austin A helium atom consists of a nucleus of charge surrounded by two electrons. Let us attempt to calculate its ground-state energy. Let the nucleus lie at the origin of our coordinate system, and let the position vectors of the two electrons be and , respectively. The Hamiltonian of the system thus takes the form. (1180)



















Post a Comment for "38 parts of a helium atom"