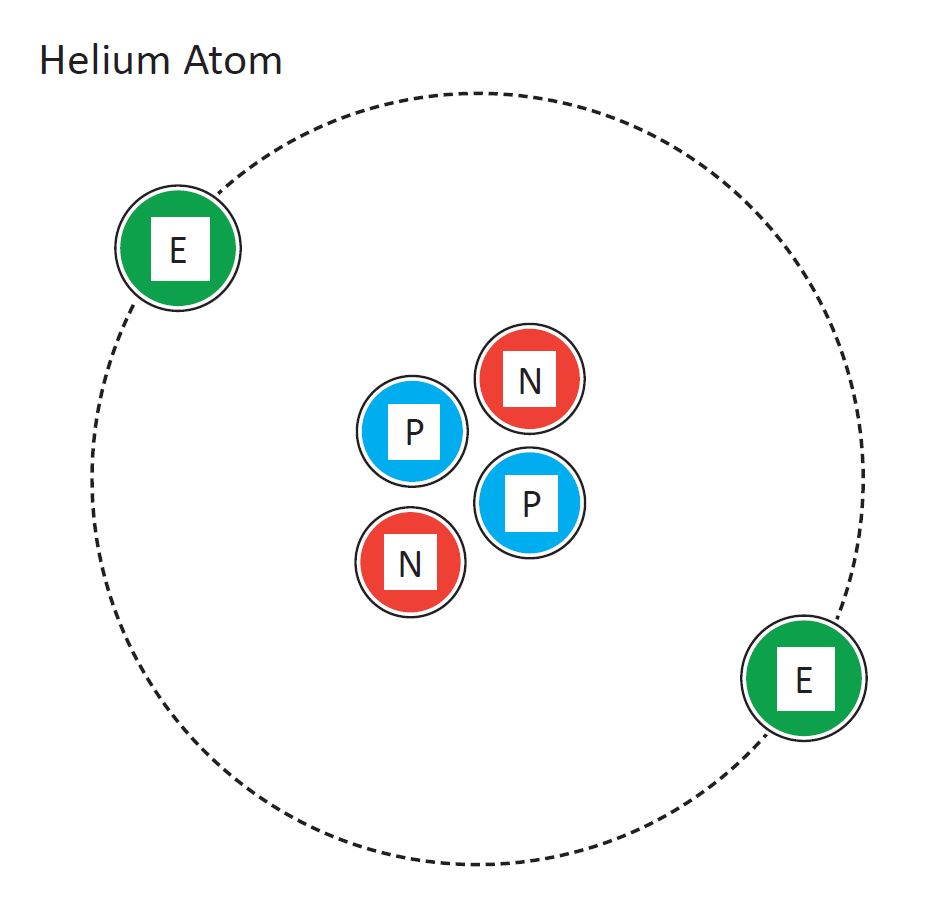
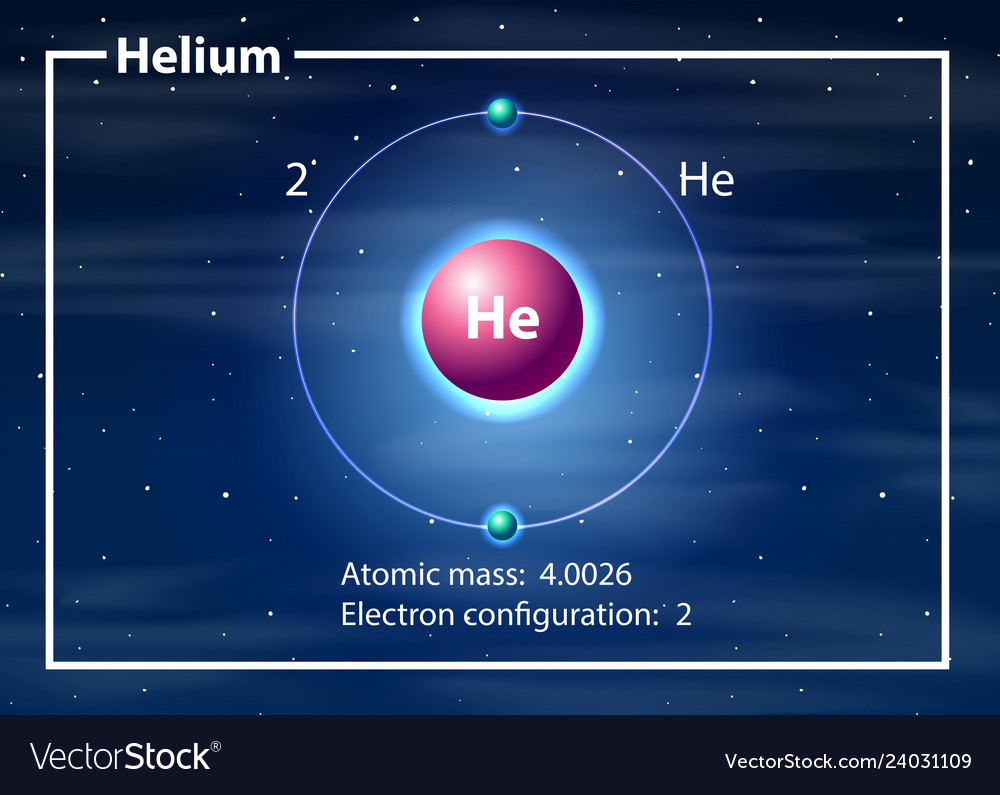
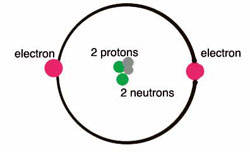
45 labeled helium atom
About: Karlsruhe - DBpedia Karlsruhe (/ˈkɑːrlzruːə/, US also /ˈkɑːrls-/, German: [ˈkaʁlsˌʁuːə] ; South Franconian: Kallsruh; formerly spelled Carlsruhe) is the second-largest city of the German federal state of Baden-Württemberg after its capital of Stuttgart, and its 313,092 inhabitants make it the 21st largest city of Germany. On the right bank of the Rhine, the city lies near the French-German border ... Helium | He - PubChem Helium | He | CID 23987 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. National Institutes of Health. National Library of Medicine. National Center for Biotechnology Information. PubChem ...
Helium - Periodic Table of Elements: Los Alamos National Laboratory Lockyer and Frankland suggested the name helium for the new element. In 1895 Ramsay discovered helium in the uranium mineral cleveite while it was independently discovered in cleveite by the Swedish chemists Cleve and Langlet at about the same time. Rutherford and Royds in 1907 demonstrated that alpha particles are helium nuclei. Sources
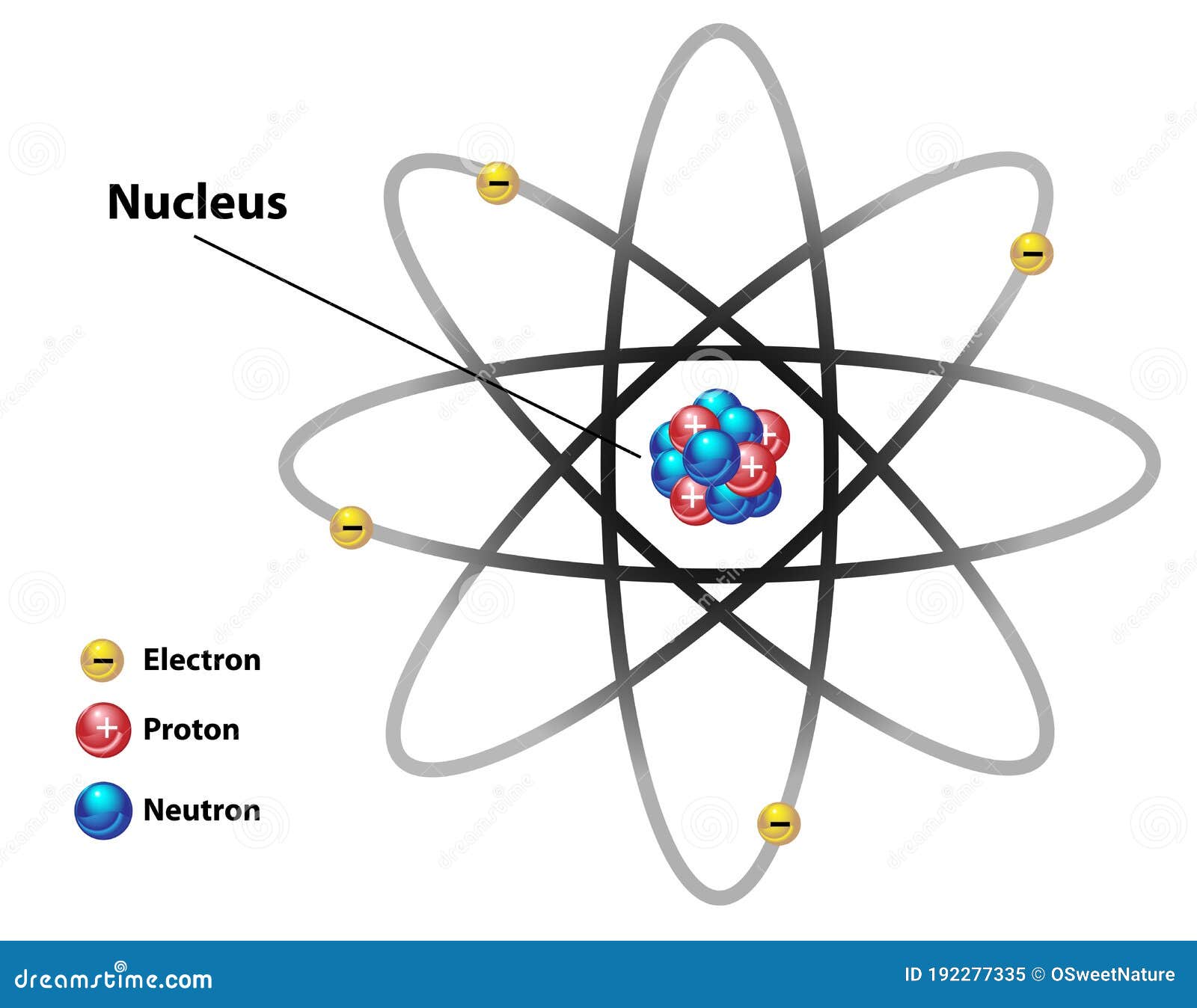
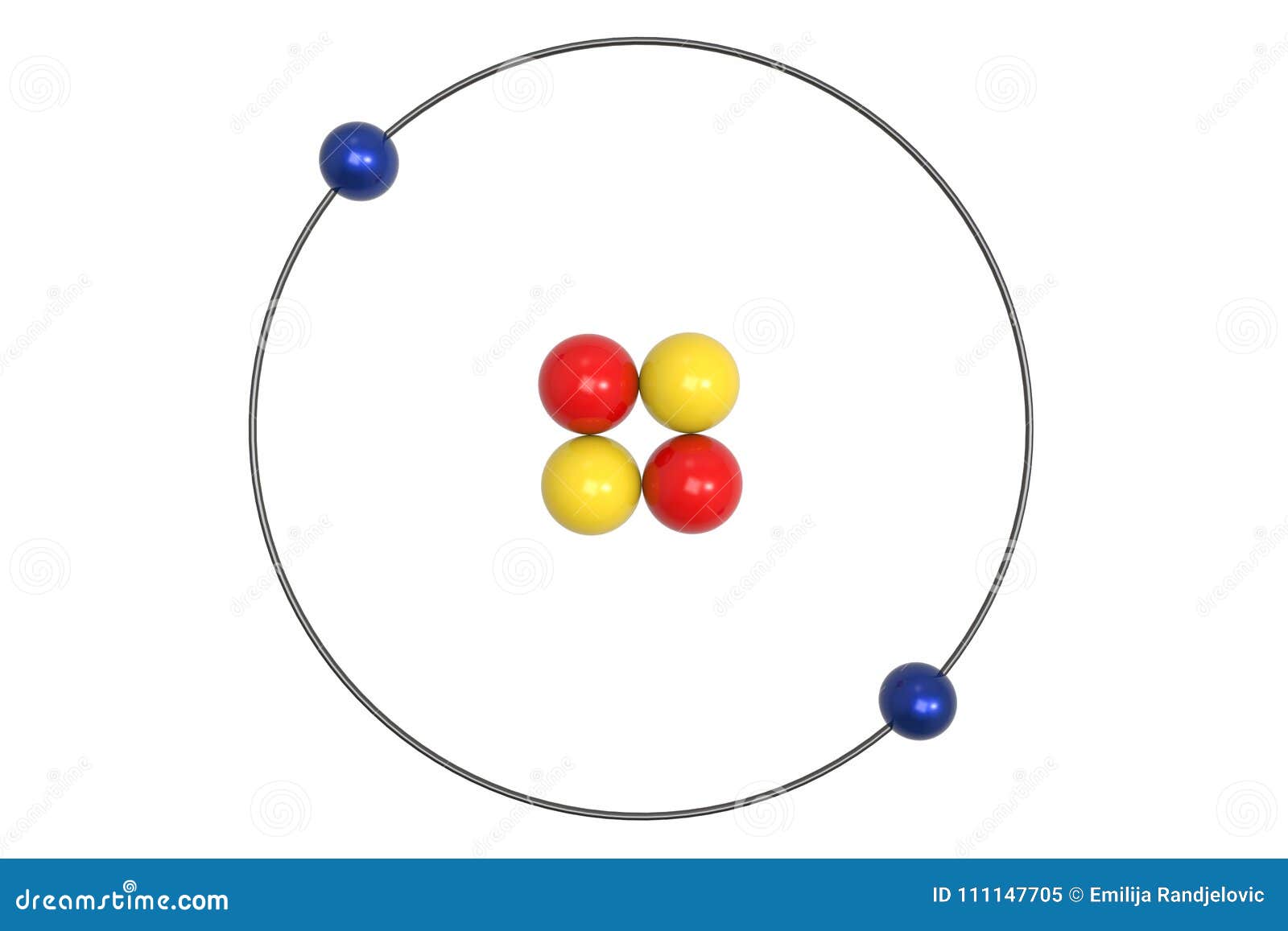
Labeled helium atom
Helium - Wikipedia Helium (from Greek: ἥλιος, romanized: helios, lit. 'sun') is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling and melting point are the lowest among all the elements.It is the second lightest and second most abundant element in the ... Helium atom - Wikipedia A helium atom is an atom of the chemical element helium. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with either one or two neutrons, depending on the isotope, held together by the strong force. Helium (He) - Periodic Table (Element Information & More) Helium is the element which has 2 electrons only, and these two electrons are nothing but they are the valence electrons. Helium element is very happy and stable with these two electrons. ( Note: Generally for all the elements, octet is the stable configuration. But helium is the only exception in which duplet configuration is stable.)
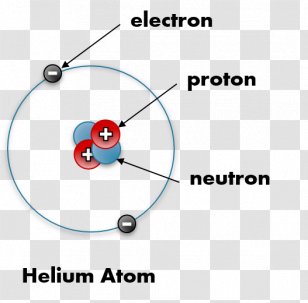
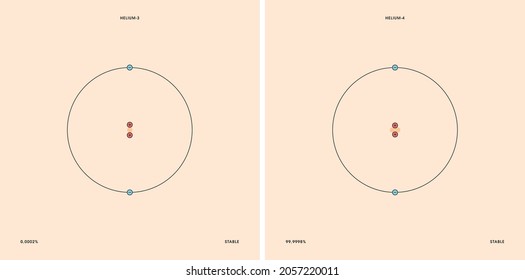
Labeled helium atom. Helium (He) - Physical & Chemical Properties, Uses, Isotopes Helium has two known stable isotopes - 3 He and 4 He. The abundance of helium-3 and helium-4 corresponds to 0.0002% and 99.9998% respectively. This difference in abundances can be observed in the Earth's atmosphere, where the ratio of 4 He atoms to 3 He atoms is approximately 1000000:1. Physical Properties of Helium Helium Atom Diagram Labeled - Bohr’s Atom | Julia Biermann Atomic structure copy and label the parts of the helium atom. That is there are 2. As shown, helium has a complete outer electron shell, . On your paper, write a description of the helium atom you just modeled. The nucleus contains uncharged neutrons and positively charged protons, whereas the orbiting electrons possess negative charges. Helium | He (Element) - PubChem Chemical element, Helium, information from authoritative sources. Look up properties, history, uses, and more. National Institutes of Health. National Library of Medicine. National Center for Biotechnology Information. PubChem. About. Posts. Submit. Contact. Search PubChem. Apologies, we are having some trouble retrieving data from our servers PDF 24. The Helium Atom - Weber State University ground state and the two lowest excited states of helium. To put these results into context, please look at the energy level diagram in Section 5.2.1 of Gri ths. This truncated-matrix approach to the helium atom, including the Mathematica code that I'll show in class, is based on a recent article by Robert C. Mass e and
helium | Definition, Properties, Uses, & Facts | Britannica helium (He), chemical element, inert gas of Group 18 ( noble gases) of the periodic table. The second lightest element (only hydrogen is lighter), helium is a colourless, odourless, and tasteless gas that becomes liquid at −268.9 °C (−452 °F). The boiling and freezing points of helium are lower than those of any other known substance. Helium plays a 'nanny' role in forming chemical ... - ScienceDaily Helium, a noble gas, was long believed to be 'too aloof' to react with the other elements on the periodic table. Now, however, scientists have provided a theoretical explanation of how helium may ... About: Baden-Württemberg Police Baden-Württemberg Police is a state law-enforcement agency in Germany. It numbers approximately 25,000 police officers and 7,000 civilian employees. The four regional police authorities (called Landespolizeidirektionen in BW) are headquartered in Karlsruhe, Stuttgart, Freiburg and Tübingen. There is also a separate police authority for the city of Stuttgart. Following a police reform in 2005 ... The helium atom - Northeastern University The helium atom has two electrons, and we will label their coordinates and , wich are combined position and spin coordinates , where the spin can assume two values . The Born-Oppenheimer Hamiltonian for the helium atom reads: (66) Since electrons are fermions, the wave function should be antisymmetric under an exchange of coordinates.
Helium - Atomic Number - He - Periodic Table Atomic Number of Helium Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. Helium - Protons - Neutrons - Electrons - Electron Configuration Helium is a chemical element with atomic number 2 which means there are 2 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. WebElements Periodic Table » Helium » properties of free atoms History. Uses. Geology. Biology. Binary compounds. Compound properties. Element reactions. Helium atoms have 2 electrons and the shell structure is 2. The ground state electron configuration of ground state gaseous neutral helium is 1s2 and the term symbol is 1S0. Helium Atom - an overview | ScienceDirect Topics The alpha particle, structurally equivalent to the nucleus of a helium atom and denoted by the Greek letterα, consists of two protons and two neutrons. It is emitted as a decay product of many radionuclides predominantly of atomic number greater than 82.
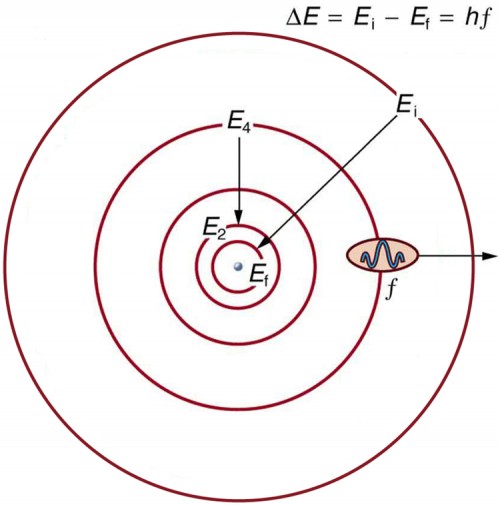
General Features of Helium States Next: The Helium Ground State Up: The Helium Atom Previous: The Helium Atom Contents. General Features of Helium States We can use the hydrogenic states to begin to understand Helium. The Hamiltonian has the same terms as Hydrogen but has a large perturbation due to the repulsion between the two electrons. ... We label the states according to ...
Helium Element | History, Uses, Facts, Physical & Chemical Characteristics Occurrence. Helium is present rarely on the Earth. However, it is the 2 nd most abundant element in the universe [2]. The atmospheric content of helium on the Earth is only 5.2 ppm [3]. There is a continuous production of helium on the Earth (via radioactive decay), but it readily escapes the Earth's atmosphere and enter the space.
Helium - Element information, properties and uses | Periodic Table Helium can be found in certain parts of the world, notably in Texas, as a minor component in some sources of natural gas. The interesting thing is how this gas gets into the ground in the first place. Unlike virtually every other atom around us, each atom of helium has been individually formed after the formation of the earth.
Helium - Periodic Table Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure.
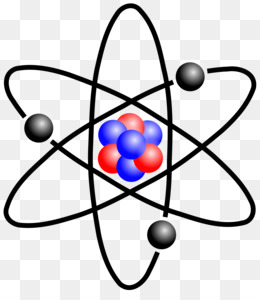
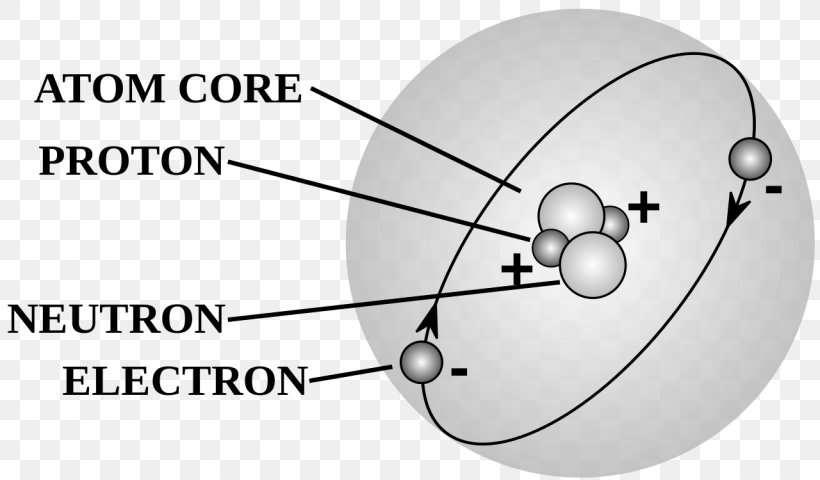
The Structure of an Atom Explained With a Labeled Diagram The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. J.J. Thomson Plum Pudding Model
Helium Atom - an overview | ScienceDirect Topics There are four possible states for the helium atom: 1 s 2 s 1 S 1 s 2 s 3 S 1 s 2 p 1 P 1 s 2 p 3 P where the S and P refer, respectively, to the total orbital angular momentum of the two electrons and the leading superscript 1 or 3 uses the multiplicity 2 S + 1 to designate the total spin.
Atom Diagrams: Electron Configurations of the Elements If there are more electrons than protons, the ion has a negative charge and is called an anion. Elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). However, it's easy to determine the configuration of electrons for heavier elements by making a chart . Hydrogen Greg Robson/CC BY 2.0 Helium Greg Robson/CC BY 2.0 Featured Video
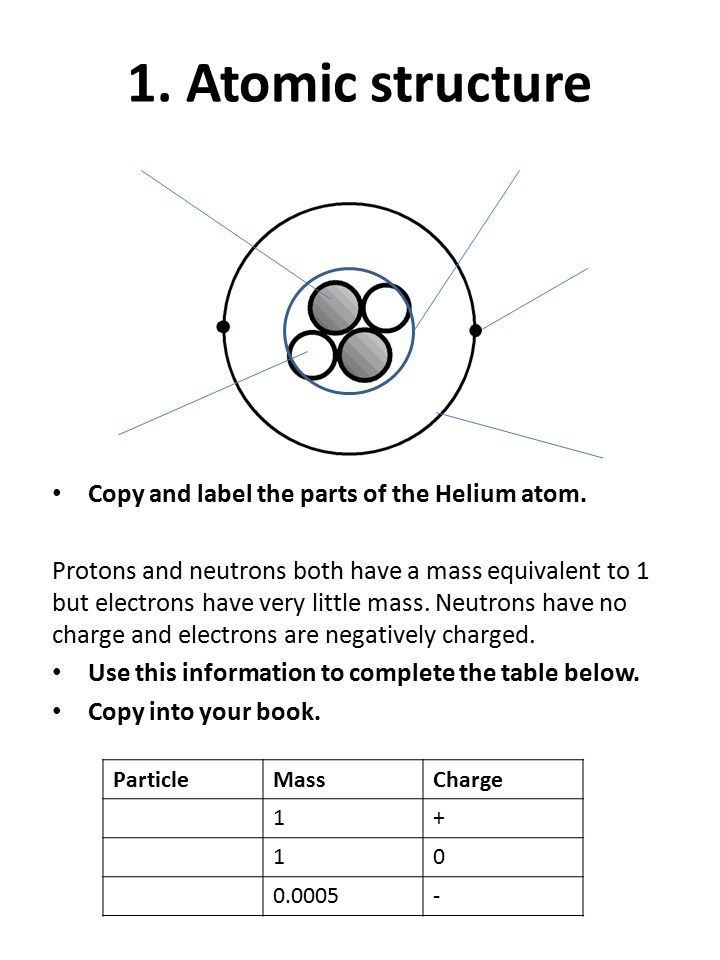
PDF Atoms Study Guide 2 He - Quia a. the atom being mostly empty space and b. the existance of a small nucleus containing most of the mass of the atom. 1. Draw and label a Helium atom: (Label the nucleus with protons & neutrons and the correct number of orbiting electrons using the symbols below) how many? N Neutrons _____ + Protons _____ -electrons _____ 2.
The diagram below shows a helium atom. The particles labeled A are ... Find an answer to your question The diagram below shows a helium atom. The particles labeled A are called (2 points) A) Electrons B) Protons C) neutrons D) nucl… danielaarevalo10 danielaarevalo10
Helium (He) - Periodic Table (Element Information & More) Helium is the element which has 2 electrons only, and these two electrons are nothing but they are the valence electrons. Helium element is very happy and stable with these two electrons. ( Note: Generally for all the elements, octet is the stable configuration. But helium is the only exception in which duplet configuration is stable.)
Helium atom - Wikipedia A helium atom is an atom of the chemical element helium. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with either one or two neutrons, depending on the isotope, held together by the strong force.
Helium - Wikipedia Helium (from Greek: ἥλιος, romanized: helios, lit. 'sun') is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling and melting point are the lowest among all the elements.It is the second lightest and second most abundant element in the ...












![Solved [Review Topics) (References] Use the References to ...](https://media.cheggcdn.com/study/d3a/d3ab1938-1726-432f-ae27-2b926a15d21e/image.png)
















Post a Comment for "45 labeled helium atom"