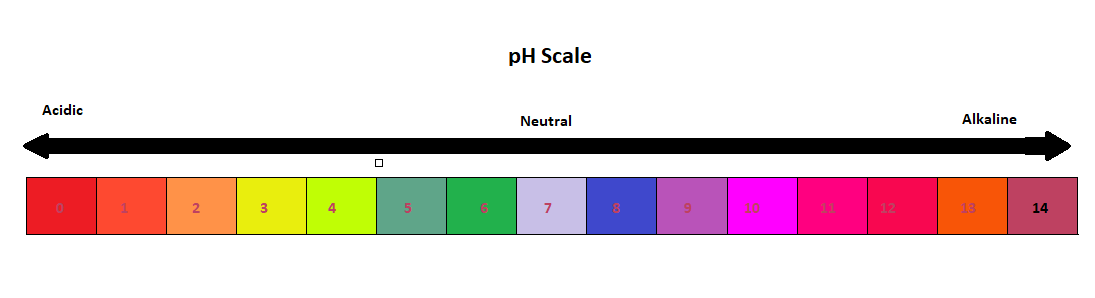
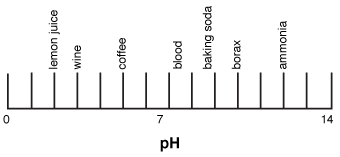
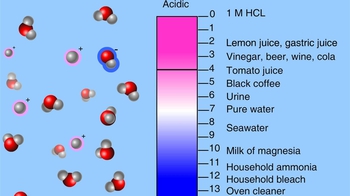
42 draw a ph scale and label water hydrochloric acid and sodium hydroxide
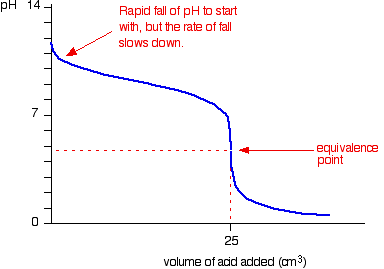
8: Acid, Bases and pH (Experiment) - Chemistry LibreTexts Then compare the color obtained to the pH scale on the instructor's desk to determine the pH value. Record these pH values to 0.1; Add 5 drops of 0.1 M (\ce{HCl}\) (hydrochloric acid) to test tubes A and B. Record the pH using pH paper. Add 5 drops of 0.1 M \(\ce{NaOH}\) (sodium hydroxide) to test tubes C and D. Record the pH using pH paper. Titration curves & equivalence point (article) | Khan Academy The solution only has salt (NaCl) and water and therefore the pH is neutral i.e. pH = 7. Point 4: Addition of NaOH continues, pH starts becoming basic because HCl has been completely neutralized and now excess of OH ^\text {-} - ions are present in the solution (from dissociation of NaOH). 2) Titration of a weak acid with a strong base
The pH Scale | Biology for Majors I - Lumen Learning The pH is calculated as the negative of the base 10 logarithm of this concentration. The log10 of 1 × 10 -7 is -7.0, and the negative of this number (indicated by the "p" of "pH") yields a pH of 7.0, which is also known as neutral pH. The pH inside of human cells and blood are examples of two areas of the body where near-neutral pH ...

Draw a ph scale and label water hydrochloric acid and sodium hydroxide
pH Scale: Acids, bases, pH and buffers (article) | Khan Academy The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although it's possible to get a pH below 0 or above 14. Anything below 7.0 is acidic, and anything above 7.0 is alkaline, or basic. Image modified from " Water: Figure 7 ," by OpenStax College, Biology, CC BY 4.0. Modification of work by Edward Stevens. Titrating sodium hydroxide with hydrochloric acid | Experiment | RSC ... Sodium hydroxide solution, 0.4 M (IRRITANT), about 100 cm 3 in a labelled and stoppered bottle Dilute hydrochloric acid, 0.4 M, about 100 cm 3 in a labelled and stoppered bottle Methyl orange indicator solution (or alternative) in small dropper bottle Health, safety and technical notes Read our standard health and safety guidance. pH scale and indicators test questions - WJEC - BBC Bitesize The pH scale is used to measure acidity and alkalinity. When an acid is neutralised, it forms a salt. ... Sodium hydroxide. 3. ... A small amount of hydrochloric acid is dissolved in a large ...
Draw a ph scale and label water hydrochloric acid and sodium hydroxide. Hydrogen and hydroxide ions - Acids and bases - BBC Bitesize Alkalis dissolve in water to give a pH greater than 7. A pH equal to 7 indicates a neutral solution. ... Hydrogen and hydroxide ions. ... Hydrochloric acid: HCl: H + (aq) Cl-(aq) Sulfuric acid: Draw a pH scale and label water hydrochloric acid and sodium hydroxide ... Draw a pH scale and label water hydrochloric acid and sodium hydroxide in their general areas on the scale 1 See answer Advertisement peterdaly See attached scan for answer which is pdf file Scan 0009 or 0.01M HCl is pH of 2, water is pH of 7 and NaOH concentrated is pH of 14. The pH Scale - Chemistry LibreTexts The pH scale expands the division between zero and 1 in a linear scale or a compact scale into a large scale for comparison purposes. In mathematics, you learned that there are infinite values between 0 and 1, or between 0 and 0.1, or between 0 and 0.01 or between 0 and any small value. PDF Acid-Base Titration Curves Using a pH Meter - gccaz.edu sodium hydroxide. You will obtain titration curves for the following combinations of acids and bases (exact concentrations will be labeled on the reagent bottles and should be written in your data table): 1) hydrochloric acid, HCl(aq) with sodium hydroxide, NaOH(aq); 2) acetic acid, CH 3 COOH(aq) with sodium hydroxide, NaOH(aq).
Biology Chapter 6 Glencoe Science Flashcards | Quizlet Ionic bond electrical attraction between two oppositely charged atoms or groups of atoms Van der Waals force attractive forces between molecules 1. Sodium has 11 protons and 11 neutrons in its nucleus. Draw a sodium atom. Be sure to label the particles ... 2. Explain why carbon monoxide (CO) is or is not an atom The pH Scale | Biology for Non-Majors I | | Course Hero The pH scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. Most parts of our body (excluding things like stomach acid) measure around 7.2 and 7.6 on the pH scale (a 7 is neutral on the scale). If foreign strong substances dramatically change this pH, our bodies can no longer function properly. pH curves (titration curves) - chemguide We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base. Running acid into the alkali For the first part of the graph, you have an excess of sodium hydroxide. The curve will be exactly the same as when you add hydrochloric acid to sodium hydroxide. Once the acid is in excess, there will be a difference. 3. Draw a pH scale and label water, hydrochloric acid, and sodium ... Draw a pH scale and label water, hydrochloric acid, and sodium hydroxide in their general areas on the scale. 2 See answers Advertisement BarrettArcher Answer: The pH scale has been drawn below. Explanation: pH sacle may be defined as the scale that determines the pH of acids and bases. 0 represent the neutral compounds.
Draw a pH scale and label water, hydrochloric acid, and sodi - Quizlet Draw a pH scale and label water, hydrochloric acid, and sodium hydroxide in their general areas on the scale. Solution Verified Create an account to view solutions By signing up, you accept Quizlet's Terms of Service and Privacy Policy Continue with Google Continue with Facebook Sign up with email Recommended textbook solutions Biology PDF SP12 1011 Titration of Hydrochloric Acid with Sodium Hydroxide Titration of Hydrochloric Acid with Sodium Hydroxide ... Draw 25.00 mL of the acid solution into the volumetric pipette and transfer this solution ... Label the pH scale below with acid, base, and ... 2.4: The pH Scale - Biology LibreTexts A solution with a high number of hydrogen ions is acidic and has a low pH value. A solution with a high number of hydroxide ions is basic and has a high pH value. The pH scale ranges from 0 to 14, with a pH of 7 being neutral. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system. The acidic reactions of ethanoic acid - RSC Education Add sodium hydroxide solution (0.4 M) to ethanoic acid and hydrochloric acid. Do this by following the procedure in steps 5, 6, 7 and 8, but using sodium hydroxide instead of sodium carbonate. Add a small piece of magnesium ribbon to the remaining hydrochloric acid tube. Try to identify the gas given off.
pH scale and indicators test questions - WJEC - BBC Bitesize The pH scale is used to measure acidity and alkalinity. When an acid is neutralised, it forms a salt. ... Sodium hydroxide. 3. ... A small amount of hydrochloric acid is dissolved in a large ...
Titrating sodium hydroxide with hydrochloric acid | Experiment | RSC ... Sodium hydroxide solution, 0.4 M (IRRITANT), about 100 cm 3 in a labelled and stoppered bottle Dilute hydrochloric acid, 0.4 M, about 100 cm 3 in a labelled and stoppered bottle Methyl orange indicator solution (or alternative) in small dropper bottle Health, safety and technical notes Read our standard health and safety guidance.
pH Scale: Acids, bases, pH and buffers (article) | Khan Academy The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although it's possible to get a pH below 0 or above 14. Anything below 7.0 is acidic, and anything above 7.0 is alkaline, or basic. Image modified from " Water: Figure 7 ," by OpenStax College, Biology, CC BY 4.0. Modification of work by Edward Stevens.


![SOLVED: QUESTION 2 [L0 MARKS] Sketch the titration curve of ...](https://cdn.numerade.com/ask_images/e94fdf91de27470899c394b96c464f26.jpg)




















Post a Comment for "42 draw a ph scale and label water hydrochloric acid and sodium hydroxide"