43 reaction coordinate diagram labeled
Description of the Reaction Coordinate - YouTube Organized by textbook: the energy versus reaction coordinate diagram. Relates the activation energies for the forward and rev... The Reaction Coordinate Diagram Questions - Chem Homework Help Draw the complete reaction mechanism including all arrows and intermediates to form the major product of the reaction. If the product is racemic label it as racemic. 9. Draw the complete reaction mechanism including all arrows and intermediates to form the major product of the reaction. Explain why this mechanism occurs the way it does. 10.
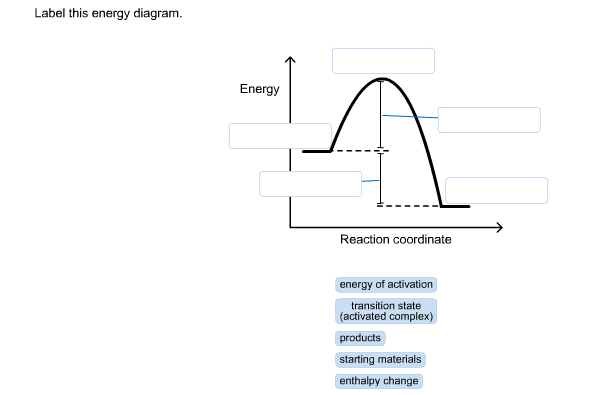
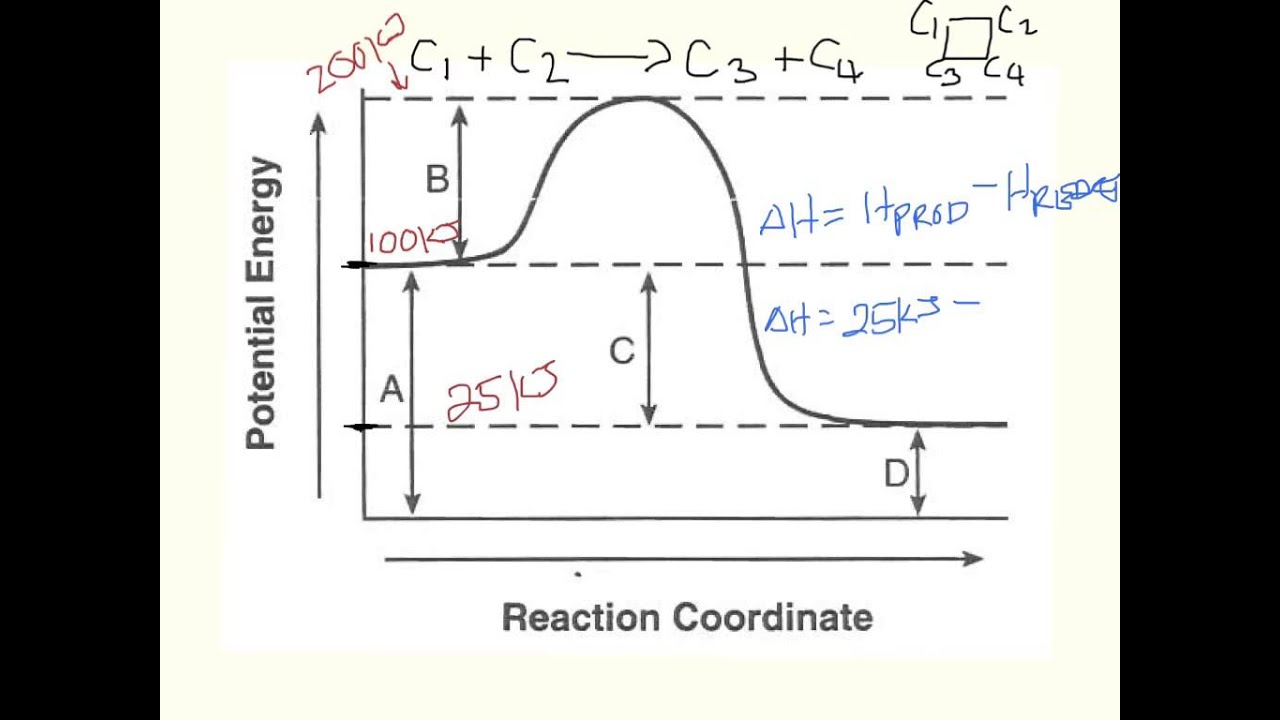
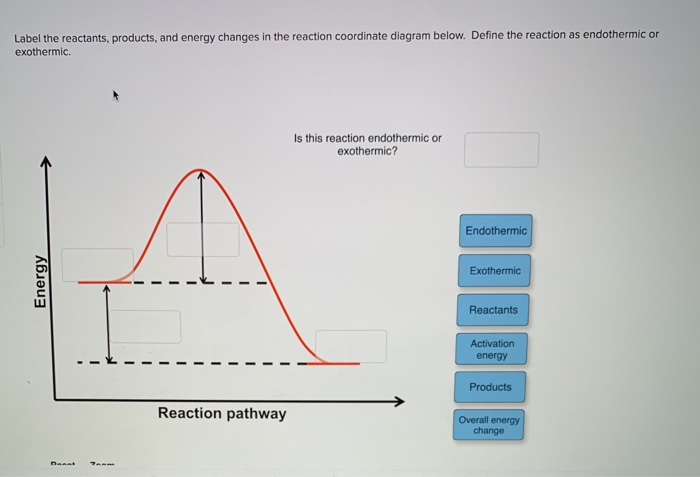
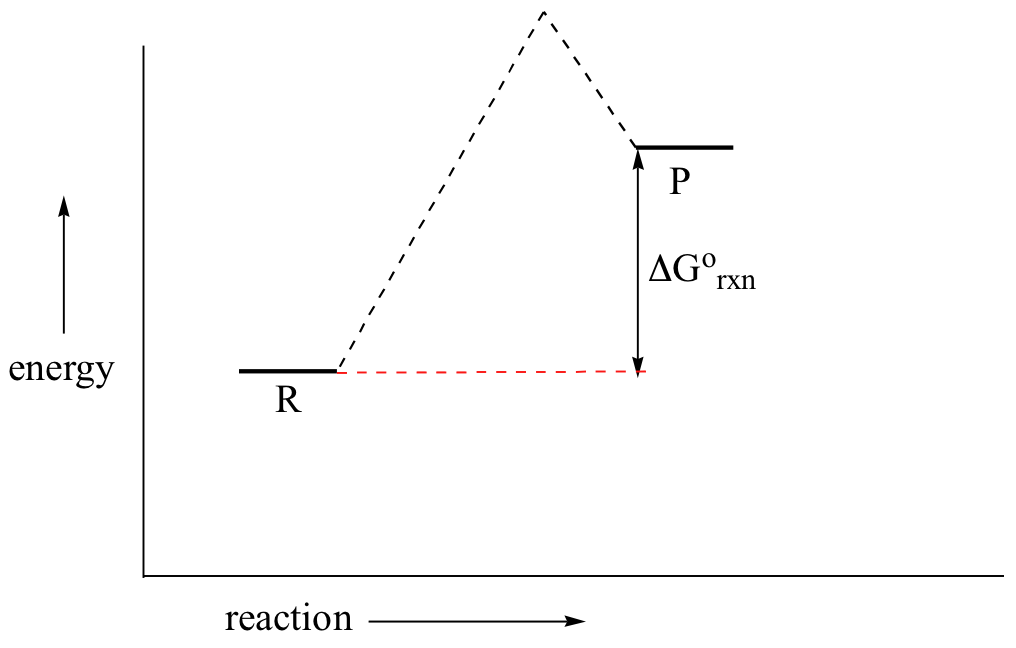
Label The Following Reaction Coordinate Diagram. - Chapter 1 ... Label the following reaction coordinate diagram enthalpy of reaction activation energy (forward) reactant (s) transition state = 0 . You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. Is the forward reaction endothermic or exothermic?

Reaction coordinate diagram labeled
chem (kinetics pt 2) Flashcards | Quizlet Label the following reaction coordinate diagram by matching between letters and numbers: (diagram in kinetics pt 2 folder in energy diagram folder on desktop) 1- J ... -Label the multi-step reaction energy diagram below using the letters corresponding to the labels on the left. There are more labels than needed; each label can be used only once. Labelthe following reaction coordinate diagram by matching ... Labelthe following reaction coordinate diagram by matching betweenletters and numbers: Answer +20. Watch. 1. answer. 0. watching. 100. views. For unlimited access to Homework Help, a Homework+ subscription is required. Jean Keeling Lv2. 10 Aug 2019. Unlock all answers. Get 1 free homework help answer. Unlock ... PDF 1. (18 points) making ethane thiolate: Derive the rate law for the ... c) Using a reaction coordinate diagram and the Hammond postulate, plot if you would expect an early or late transition state for the first step (A to B) of this reaction. Label this plot "C". (2 points) PE Reaction coordinate d) Based on your answer to part (c), what range would you expect the β LG values fall within? Explain your answer.
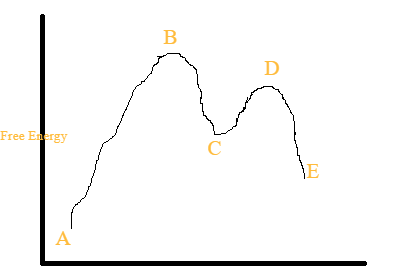
Reaction coordinate diagram labeled. How can I draw a reaction coordinate in a potential energy diagram? 1 Answer. Pratik B. Dec 1, 2014. The graph of reaction co-ordinate vs potential energy for standard exothermic and endothermic reactions are known. Reaction co-ordinates represent the way the reactant molecules "evolve" to give products.These plots can be computed by using softwares like Gaussian. (I use it regularly) SOLVED:a. Draw and label the reaction coordinate diagram for reactions ... draw and label the reaction coordinate diagram for reactions below, including for each reaction: the transition states, activation energy, reactants, intermediates, products, and axes, as applicable. (6 points) a) b) cl b which reaction is most likely to proceed by the mechanism shown? (1 point) justify your answer in part b (causal explanation), … 6.6: Reaction Coordinate Diagrams - Chemistry LibreTexts In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this: Analyzing Energy With a Reaction Coordinate Diagram - Study.com So let's look a little closer at the reaction coordinate diagram and what each point means: Let's start at 'A'. This is the reactants before the reaction has occurred. As the reaction occurs,...
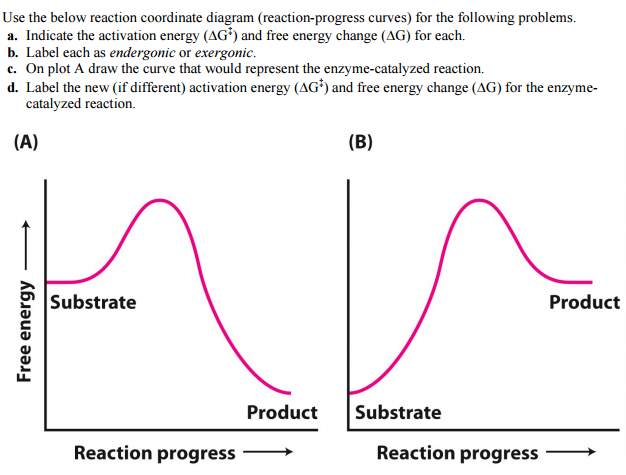
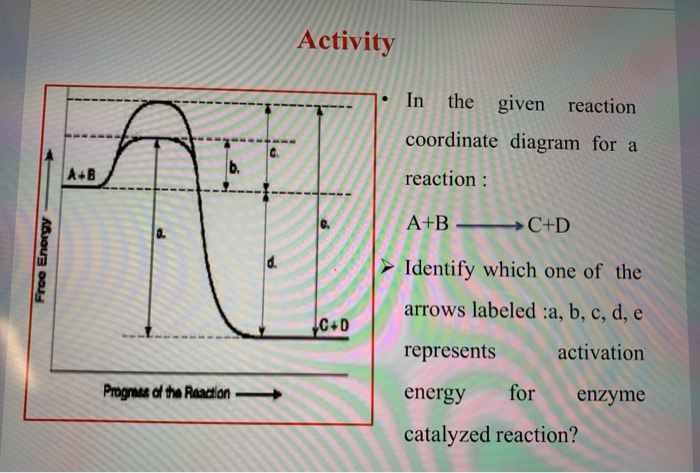
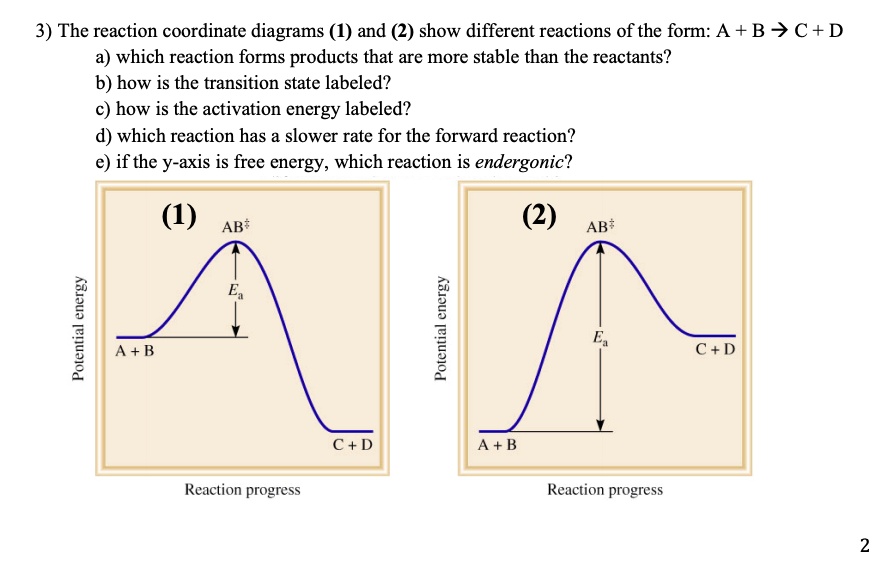
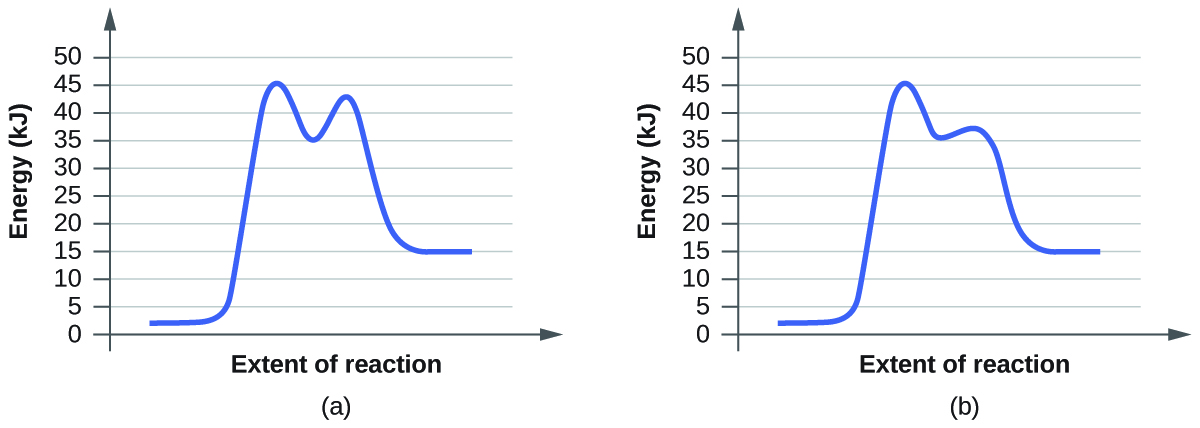
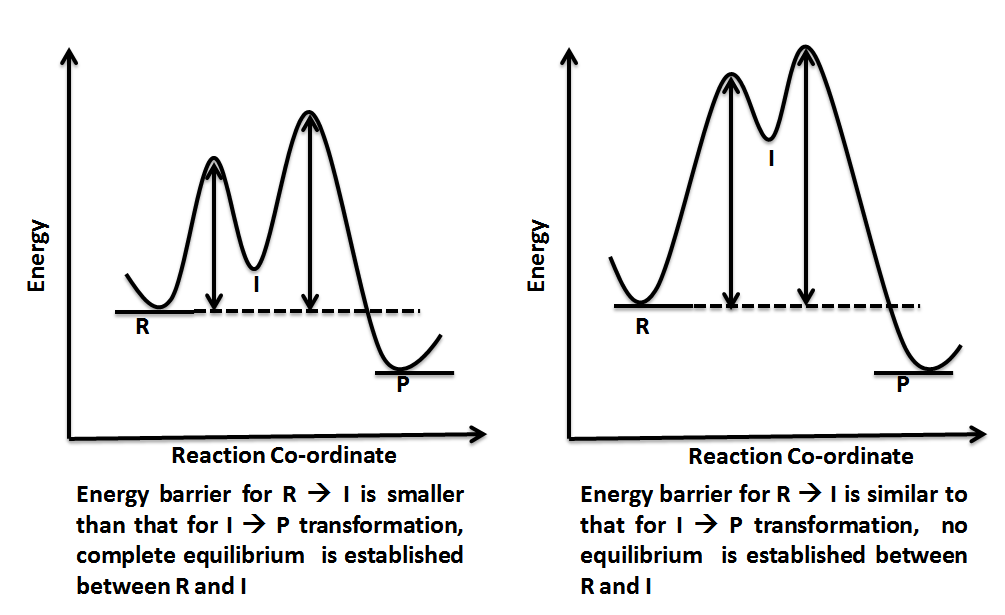
OneClass: Given the reaction coordinate diagram for the reaction of A ... Draw a properly labeled reaction coordinate diagram for a reaction with the following. criteria: make sure to clearly indicate Î G and any activation energies (for the forward reaction) as well as all intermediates and transition states. a) exergonic 3 step reaction. b) the first step is the rate-determining step Answered: 1. a) Write the reaction profile… | bartleby 1. a) Write the reaction profile (reaction coordinate diagram) for an endothermic reaction that occurs via a 3 step mechanism with the second step being the rate determining step? b) Label reactants and products, each activation energy and the enthalpy change for the reaction. c) Label the location of intermediates. Saddle point diagram - Big Chemical Encyclopedia A two-dimensional "slice" through a saddle point diagram is typically called a reaction-coordinate diagram or potential-energy profile. [Pg.625] The bifurcational diagram (fig. 44) shows how the (Qo,li) plane breaks up into domains of different behavior of the instanton. In the Arrhenius region at T> classical transitions take place throughout ... Label The Following Reaction Coordinate Diagram / Consider The Energy ... A graph is shown with the label, "reaction coordinate," on the x figure 1. Label the following reaction coordinate diagram. Label the following reaction coordinate diagram enthalpy of reaction activation energy (forward) reactant (s) transition state = 0 . Labeling parts of a reaction coordinate diagram.
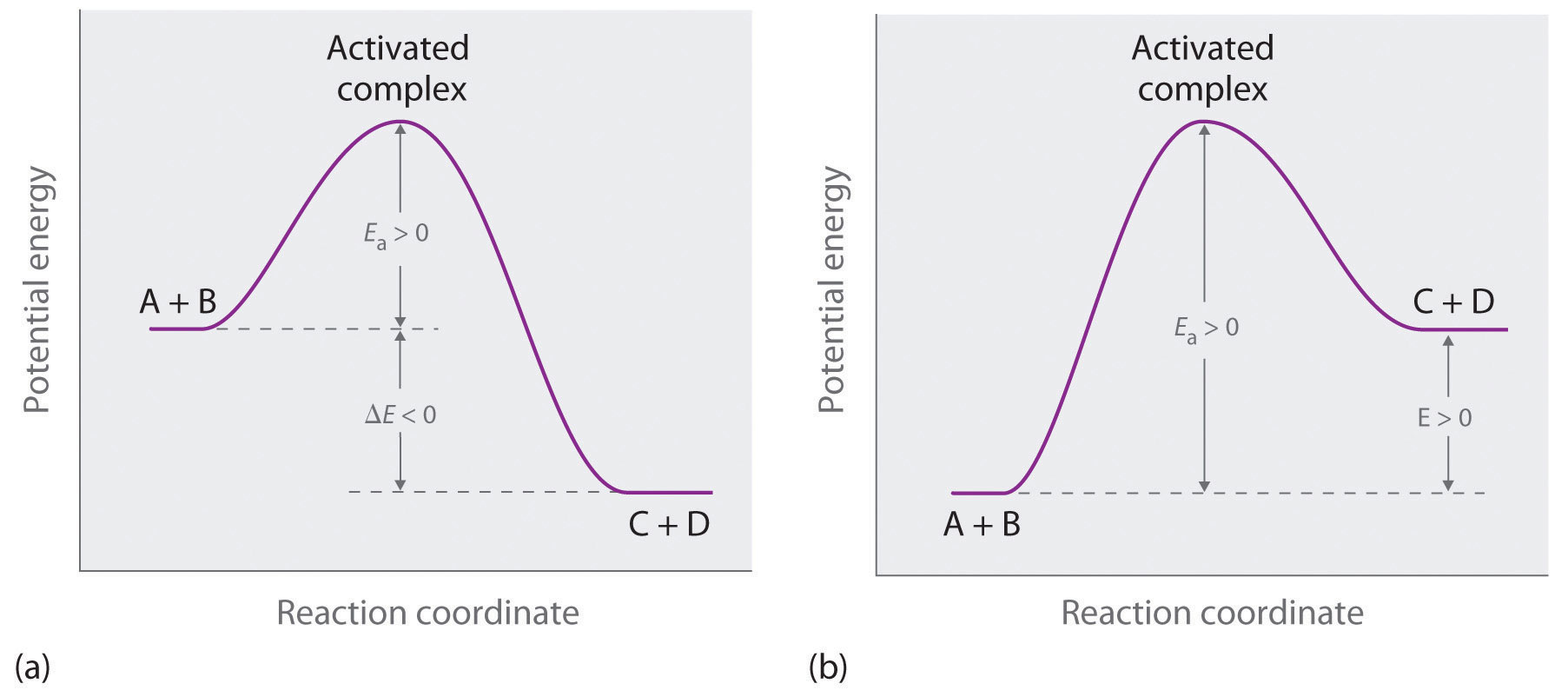
Reaction Coordinate Diagrams The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction (heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram. Solved Label the following reaction coordinate diagram. - Chegg Label the following reaction coordinate diagram. Energy Reactant (s) Transition State Product (s) Activation Energy (forward) Transition State Activation Energy (forward) Energy Enthalpy of Enthalpy of Reaction Product (s) Reaction AHrxn Reactant (s) Reaction Coordinate Reaction Coordinate Reset Zoom Draw a reaction coordinate diagram for a two-step reaction i - Quizlet Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states. Explanation Verified Reveal next step Reveal all steps Create a free account to see explanations Answered: Collision Theory: Reaction Coordinate… | bartleby Question. Transcribed Image Text: Energy Energy 个 Collision Theory: Reaction Coordinate Diagram for Exothermic Process (forward direction) Thermochemistry Collision Theory Reactants Products Reactants Products Activation Energy:, Activated Complex Diagram: AB + C A + BC.
High School Chemistry: Reaction Rates & Equilibrium - Rapid Learning Center Reaction Coordinate Diagrams Reaction coordinate diagrams show the energy of the reactants, the activation energy up to the activated complex, or transition state (the in-between state between the reactants and the products), and the energy of the products. The overall energy change of the reaction is also shown. Factors affecting rate
Reaction Coordinates in Potential Energy Diagrams - Chemistry ... Reaction Coordinates in Potential Energy Diagrams Reaction potential energy diagrams are graphs that show the energy of a process as a function of the extent to which that process has occurred. As these are graphs showing mathematical functions, there must be a numerical coordinate axis that shows the independent variable. This coordinate is called the reaction coordinate, and it reflects the ...
Reaction Coordinate Diagrams - College Chemistry - Varsity Tutors Explanation: The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants.
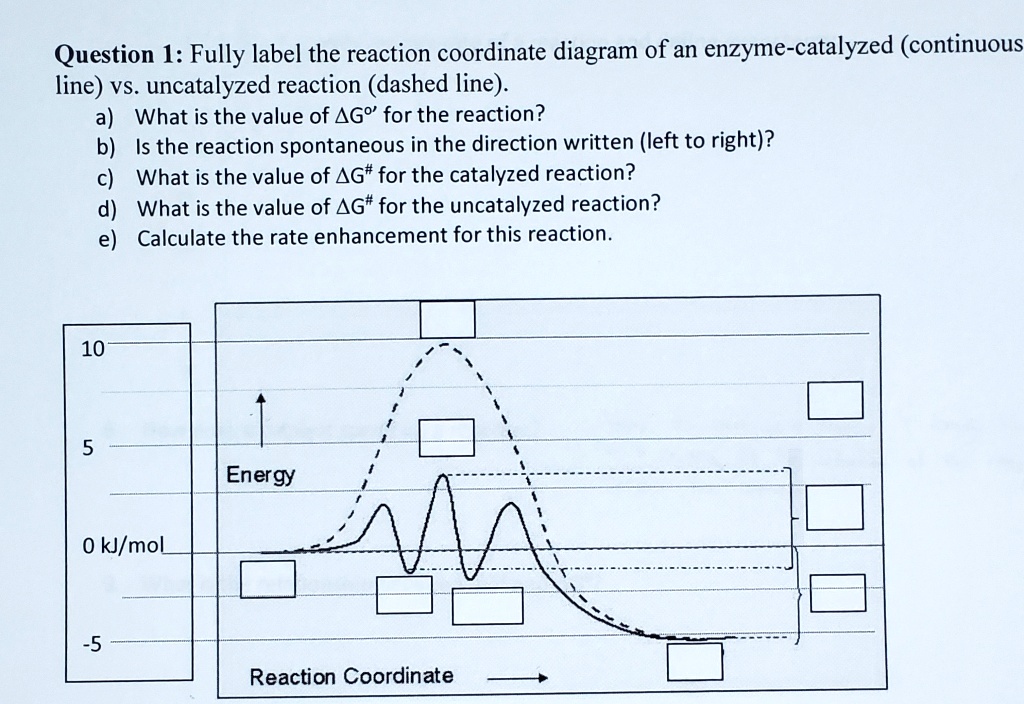
12.7 Catalysis - Chemistry - opentextbc.ca One such reaction is catalytic hydrogenation, the process by which hydrogen is added across an alkene C=C bond to afford the saturated alkane product. A comparison of the reaction coordinate diagrams (also known as energy diagrams) for catalyzed and uncatalyzed alkene hydrogenation is shown in Figure 1. Figure 1.
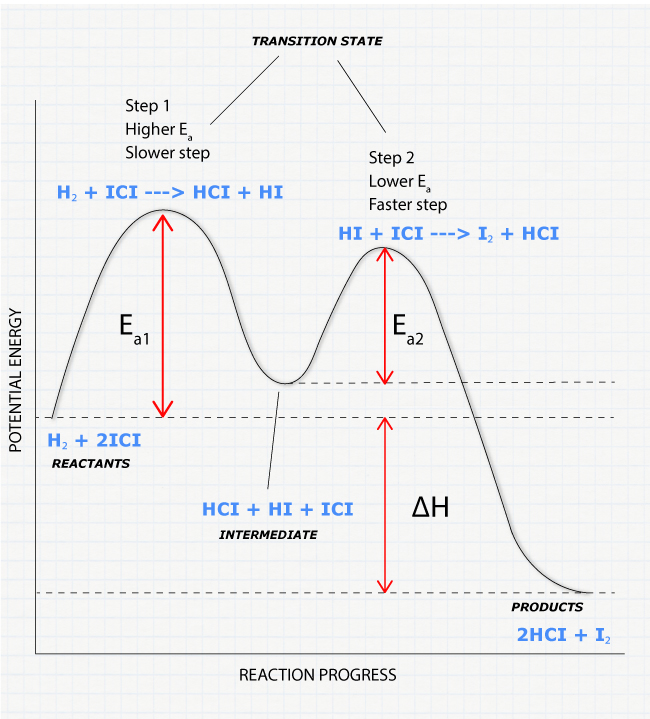
6. Reaction Coordinate Diagram - High School/Honors/AP® Chemistry ... Reaction Coordinate Diagram Given the following reaction, sketch a reaction coordinate graph. The reaction involves two steps, step 1 is the slowest step and step 2 is the fastest step. Both steps are exothermic. Indicate on the diagram the overall enthalpy change of the reaction, the reaction for the transition states and intermediate states.
Labeling Parts of a Reaction Coordinate Diagram - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
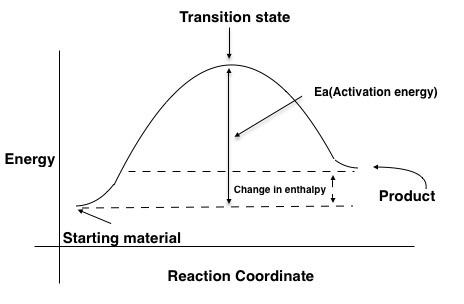
PDF Teacher's Guide to "Visualizing the Transition State and Reaction ... A reaction energy diagram (Figure 1) is presented on the chalk board (complete with axes labeled: potential energy vs. reaction coordinate (or reaction progress)). The activation energy, Ea, (the change in energy from reactants to the top of the "hill") is labeled.
How can I draw activation energy in a diagram? | Socratic 1. Draw and label a pair of axes. Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4.
Arrhenius Theory and Reaction Coordinates - Chemistry 302 A reaction coordinate is a path that links the reactant molecules and the products molecules. In many reactions, we can directly envision this coordinate as the length of a particular bond or bonds. In other cases, the reaction coordinate is used merely to represent some unknown coordinate.
PDF Energy/Reaction Coordinate Diagrams - chemconnections A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change ... • The reaction rate (the number of effective collisions in a period of time) relates to many factors. ... Label each of those atoms nucleophilic (nu) or electrophilic (el) in each resonance structure.! ...
PDF 1. (18 points) making ethane thiolate: Derive the rate law for the ... c) Using a reaction coordinate diagram and the Hammond postulate, plot if you would expect an early or late transition state for the first step (A to B) of this reaction. Label this plot "C". (2 points) PE Reaction coordinate d) Based on your answer to part (c), what range would you expect the β LG values fall within? Explain your answer.
Labelthe following reaction coordinate diagram by matching ... Labelthe following reaction coordinate diagram by matching betweenletters and numbers: Answer +20. Watch. 1. answer. 0. watching. 100. views. For unlimited access to Homework Help, a Homework+ subscription is required. Jean Keeling Lv2. 10 Aug 2019. Unlock all answers. Get 1 free homework help answer. Unlock ...
chem (kinetics pt 2) Flashcards | Quizlet Label the following reaction coordinate diagram by matching between letters and numbers: (diagram in kinetics pt 2 folder in energy diagram folder on desktop) 1- J ... -Label the multi-step reaction energy diagram below using the letters corresponding to the labels on the left. There are more labels than needed; each label can be used only once.




















Post a Comment for "43 reaction coordinate diagram labeled"