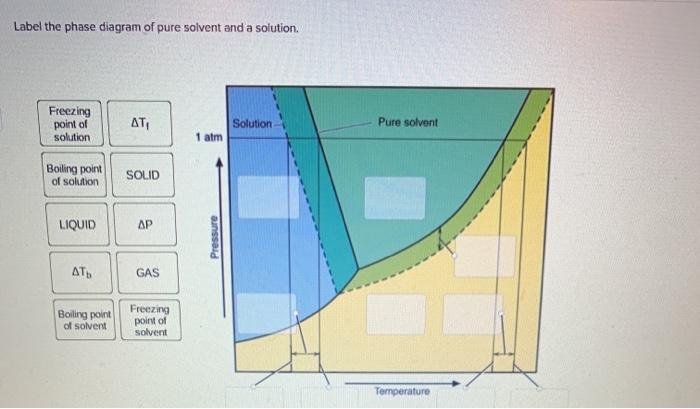
40 label the phase diagram of pure solvent and a solution.
13.6: Vapor Pressures of Solutions - Chemistry LibreTexts Feb 02, 2022 · Given: identity of solute, percentage by mass, and vapor pressure of pure solvent. Asked for: vapor pressure of solution. Strategy: Calculate the number of moles of ethylene glycol in an arbitrary quantity of water, and then calculate the mole fraction of water. Use Raoult’s law to calculate the vapor pressure of the solution. Solution: PDF Phase Diagrams When a second compound is introduced to the system forming a homogeneous solution however, the phase diagram drastically changes. For example, the addition of a solute to a pure solvent (making a solution) can disrupt important interactions between solvent molecules, changing the temperature at which the solvent would typically freeze or boil.
57 Features of Phase Diagrams (M11Q1) - Unizin A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2.

Label the phase diagram of pure solvent and a solution.
The figure shows two phase diagrams, one for a pure ... - Socratic.org Similarly, the normal boiling point of the pure substance at constant pressure is indicated by C, and the raised normal boiling point of the solution with the pure liquid as the solvent (again, due to addition of nonvolatile solute to the pure liquid substance) is indicated by D, since T ↑ rightwards. ΔT b = T b − T * b = iKbm Phase Diagrams | Chemistry | | Course Hero A typical phase diagram for a pure substance is shown in Figure 1. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2. The pressure and temperature axes on this phase diagram of water are not drawn to constant scale in order to illustrate several important properties. The phase diagram for solvent and solutions is shown in the figure ... The phase diagram for solvent and solutions is shown in the figure. What represents the normal boiling point of the solution? A A B B C C D D Hard Solution Verified by Toppr Correct option is D) The normal boiling point of the solution is that temperature at which vapour pressure of solution equals to 1 atm.
Label the phase diagram of pure solvent and a solution.. The phase diagrams for the pure solvent (solid lines) and the solution ... The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below: The quantity indicated by `L` i... 13.8: Freezing-Point Depression and Boiling-Point Elevation ... Feb 03, 2022 · We can see why this must be true by comparing the phase diagram for an aqueous solution with the phase diagram for pure water (Figure \(\PageIndex{1}\)). The vapor pressure of the solution is less than that of pure water at all temperatures. Consequently, the liquid–vapor curve for the solution crosses the horizontal line corresponding to P ... Ternary Phase Diagram - an overview | ScienceDirect Topics A ternary phase diagram shows possible phases and their equilibrium according to the composition of a mixture of three components at constant temperature and pressure. Figure 4.23 shows a schematic of a ternary phase diagram. Single-phase regions are areas that originate from the vertex of the triangle and that are not enclosed by black curves. Answered: 1) Label the LLE diagram provided… | bartleby 1) Label the LLE diagram provided indicating which component is the solvent and which is cariet, and identify the solvent rich (extract) phase boundary and the raffinate phase boundary. 2) Identify Each of the (mark as 'a', 'b', etc) following points and indicate types and number of phases present in each composition. a.
PDF Chapter 9: Phase Diagrams - Florida International University Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash Chemical Phase Equilibria and Phase Diagrams | SpringerLink At low temperatures, the equilibrium state is a mixture of pure A and B, i.e., there is no mutual solubility between A and B. At intermediate temperatures, depending on the composition, the equilibrium states can be a mixture of pure A and liquid solution, a two-phase mixture of pure B and liquid solution, or a single-phase liquid solution. 10.4 Phase Diagrams - General Chemistry 1 & 2 A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2. Answered: The phase diagrams for a pure solvent… | bartleby identify the normal freezing (fpsolv) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpgoln) points of the solution at 1 atm. assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent. 1 atm liquid solid answer bank fpsolv bpsolv fpsoln bpsoln gas temperature …
Phase Diagrams - Chemistry 2e - opentextbc.ca A typical phase diagram for a pure substance is shown in (Figure). The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in (Figure). Phase diagram - Wikipedia A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium . Contents 1 Overview 2 Types 2.1 2-dimensional diagrams PDF Chapter Phase Diagrams - Uttar Pradesh Textile Technology Institute A phase may be defined as a homogeneous portion of a system that has uniform phys- ical and chemical characteristics. Every pure material is considered to be a phase; so also is every solid, liquid, and gaseous solution. For example, the sugar-water syrup solution just discussed is one phase, and solid sugar is another. Phase Diagram | Explanation, Definition, Summary & Facts The phase diagram of a substance can be used to identify the physical and chemical properties of that substance. Here, we will study a general phase diagram by considering different values of one variable while keeping the other variable value constant. In a phase diagram temperature values are drawn on x-axis, whereas pressure values on y-axis.
Phase Diagrams - 2012 Book Archive The Phase Diagram of Water. Figure 11.23 "Two Versions of the Phase Diagram of Water" shows the phase diagram of water and illustrates that the triple point of water occurs at 0.01°C and 0.00604 atm (4.59 mmHg). Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point (273.16 K) is used to define the ...
Chemistry, Unit 6 Flashcards - Quizlet 18) The difference between the boiling point of a pure solvent and the boiling point of a solution of a nonelectrolyte in the same solvent is called the _____, a colligative property. 19) _______ properties depend on the concentration of a solute in a solution but not on the identity of the solute.
Solved Label the phase diagram of pure solvent and a - Chegg Question: Label the phase diagram of pure solvent and a solution Freezing point of solution GAS Solution Pure solvent 1 atm Boiling pointFreezing point of of solvent solvent ??? 11 SOLID AT AP Boiling pointLIQUID of solution Temperature This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (10 ratings)
10.4 Phase Diagrams – Chemistry (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 10 10 Pa, it is converted into diamond. Label the diamond phase. (d) Circle each triple point on the phase diagram.
Solved Label the phase diagram of pure solvent and a - Chegg Science. Chemistry. Chemistry questions and answers. Label the phase diagram of pure solvent and a solution.
PDF Binary Solid-Liquid Phase Diagram Introduction - Williams College Binary Solid-Liquid Phase Diagram Introduction The substances that we encounter in the material world are hardly ever pure chemical compounds but rather mixtures of two or more such compounds. The individual substances in such a mixture may behave more or less independent of each other but merely diluted, i.e., an ideal solution or
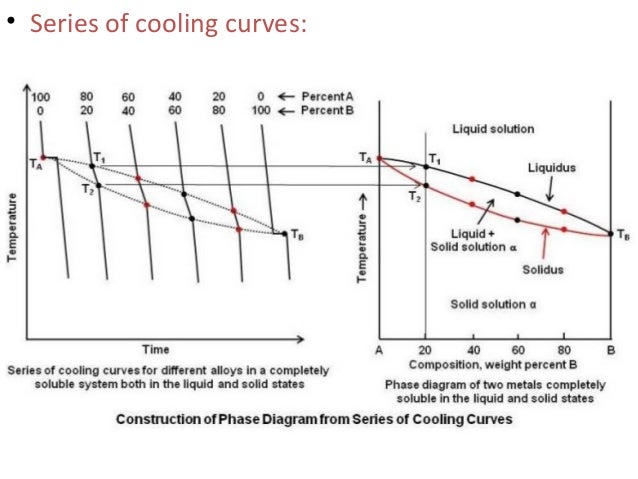
Phase Diagram - SlideShare - The phase diagram shows two distinct phases; one is liquid metal solution and the other is solid solution. - Liquidus is that line - Above which the alloy is in liquid state - Where solidification starts - Solidus is that - Below which the alloy is in solid state, and - Where the solidification completes.
Liquid/Solid Phase Diagram - an overview | ScienceDirect Topics The (solid + liquid) phase diagram for ( x1 n-C 6 H 14 + x2 c-C 6 H 12) has a eutectic at T = 170.59 K and x2 = 0.3317. A solid phase transition occurs in c-C 6 H 12 at T = 186.12 K, resulting in a second invariant point in the phase diagram at this temperature and x2 = 0.6115, where liquid and the two solid forms of c-C 6 H 12 are in equilibrium.



Post a Comment for "40 label the phase diagram of pure solvent and a solution."